Metal corrosion refers to the degradation of materials caused by the reaction between metal and its environment. It is an electrochemical process, where any condition that facilitates electron flow will accelerate corrosion.
1.Types of Metal Corrosion
Depending on the corrosion environment and metal type, corrosion can be classified into three forms: discoloration, corrosion, and rust.
- Discoloration
Discoloration is a mild form of surface corrosion that results in color changes or loss of luster. It generally does not affect the structural integrity of the metal but only its appearance. Metals like copper and silver can discolor in environments with oxygen, sulfur, or halogens, forming oxides or sulfides, especially in humid conditions.
- Corrosion
Corrosion involves more extensive damage and changes in metallurgical properties. For example, when aluminum or zinc corrodes, it forms a white powder, and copper forms a green layer.
- Rust
Rust is the corrosion of ferrous metals like iron, resulting in the formation of iron oxides, which is the most common type of corrosion.
2.Mechanism of Common Metal Corrosion
The essence of corrosion is the exchange of electrons between the metal surface and its environment. In this process, the metal is oxidized, leading to electrochemical reactions. For instance, in the corrosion of iron with water droplets, a concentration cell is formed, causing corrosion. The oxygen content at the center of the water droplet is lower than at the edges, creating an anode and cathode region, leading to the formation of rust.
Mechanism of Rust Formation on Iron Surface
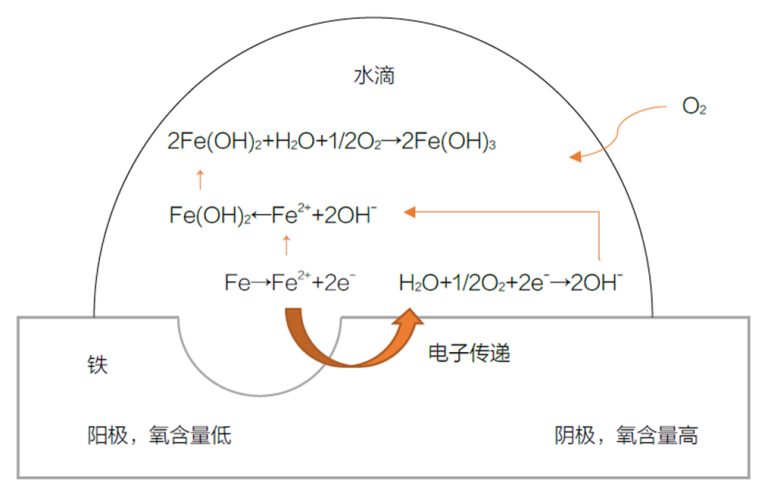
- The low oxygen region acts as the anode, where iron atoms lose electrons and become iron ions: Fe → Fe²⁺ + 2e⁻
- At the water droplet’s edge, water and the released electrons reduce oxygen, forming hydroxide ions (the cathode region): ½ O₂ + H₂O + 2e⁻ → 2OH⁻
- The hydroxide ions combine with iron ions to form ferrous hydroxide: Fe²⁺ + 2OH⁻ → Fe(OH)₂
- Ferrous hydroxide further reacts with water and oxygen, forming brown rust: 2Fe(OH)₂ + H₂O + ½ O₂ → 2Fe(OH)₃
3.Types of Corrosion
3.1 Uniform Corrosion
When water or other liquids fully cover a metal surface, the anode and cathode regions shift continuously, leading to uniform corrosion. This is the most common form of everyday metal corrosion.
3.2 Electrolyte Effects
Pure water has low corrosion potential due to its low ionization capacity. However, adding acids, bases, or salts increases ion concentration, accelerating corrosion. In some cases, electrolytes react with the metal surface to form a passivation film, slowing down the corrosion process.
3.3 Oxygen Concentration Corrosion
As described earlier, the low oxygen region acts as the anode, and the high oxygen region acts as the cathode, causing oxygen concentration cell corrosion.
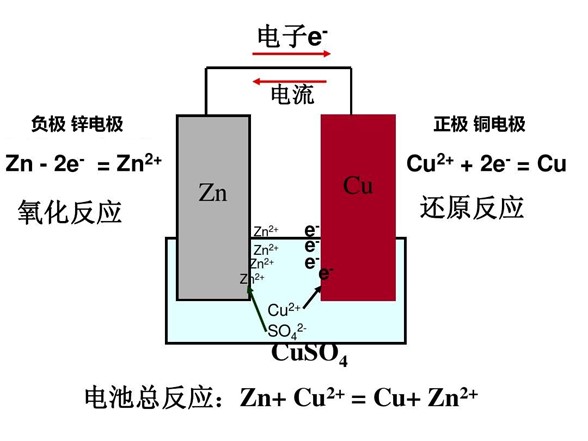
3.4 Galvanic Corrosion
When different metals are immersed in the same liquid, an electrochemical reaction occurs, causing galvanic corrosion. This is the principle behind batteries.
Principle of Battery
3.5 Erosion Corrosion
Erosion corrosion occurs when fast-flowing liquids mechanically remove the metal surface. This is particularly important in pipelines and pump systems.
3.6 Fretting Corrosion
Similar to erosion, fretting corrosion occurs when solid materials remove protective films from metal surfaces, commonly seen in contact points between parts.
3.7 Pitting Corrosion
Pitting corrosion happens when small areas of a metal surface experience localized corrosion. For example, when a protective oxide layer is scratched, the exposed area becomes an anode, leading to a corrosion pit.
3.8 Intergranular Corrosion
Intergranular corrosion is a type of localized corrosion that spreads along the grain boundaries of metals. It occurs in stainless steel when heated to certain temperatures, creating chromium-depleted zones at the grain boundaries, leading to loss of corrosion resistance.
3.9 Stress Corrosion
Stress corrosion occurs under tensile stress, especially in welded workpieces with residual stress. The protective film on the metal surface is damaged, leading to cracks that propagate over time, causing fractures.
3.10 Microbial Induced Corrosion
Microorganisms, such as aerobic and anaerobic bacteria, in water-based metalworking fluids can cause microbial-induced corrosion. Aerobic bacteria degrade long-chain molecules in lubricants, forming acids that cause corrosion, while anaerobic bacteria produce sulfur compounds, leading to discoloration.
Bacterial Respiration

4.Corrosion Prevention Countermeasures
- Passivating Agents
Metals can form a natural oxide layer for passivation, or specific salts can be used to create a protective film on the metal surface. For instance, iron can be passivated by immersion in nitrites, chromates, or molybdates.
- Organic Films
Organic films, such as oil-based coatings, can be applied to metal surfaces, forming a barrier between the metal and its environment. However, this method provides only temporary protection.
- Bacterial Inhibitors
If corrosion is caused by bacteria, bactericides can be used to inhibit aerobic bacteria, while oxygen can be added to control anaerobic bacteria. If microbial corrosion is already extensive, the working fluid may need to be replaced to prevent further damage.

